Scientific Leadership
The AACR uses its broad scientific scope and the vision of its brain trust within the membership to identify scientific priorities and also the obstacles to progress. Our organization fosters collaboration among scientists, clinicians, population scientists, and advocates to overcome the obstacles and thereby reduce cancer incidence, morbidity, and mortality; increase survival from cancer; improve the lives of patients with cancer; and increase cancer prevention.
AACR Project GENIE: Powering Precision Medicine
Precision medicine requires an end-to-end learning health care system, wherein the treatment decisions for patients are powered by the prior experiences of similar patients. Oncology is currently leading the way in precision medicine because the factors that fuel cancer initiation, progression, and recurrence—the genomic and other molecular characteristics of patients and their tumors—are routinely collected at scale. A major challenge to realizing the promise of precision medicine is that no single institution is able to sequence and treat sufficient numbers of patients to improve clinical decision-making independently, which is why the AACR Project GENIE (Genomics Evidence Neoplasia Information Exchange) registry was established.
AACR Project GENIE is an international pan-cancer registry of real-world data assembled through data sharing among 19 leading international cancer centers. The registry pools clinical sequencing data from participating cancer centers to create an evidence base that is available to everyone. The consortium and its activities are driven by openness, transparency, and inclusion, ensuring that the project output remains accessible to the global cancer research community for the benefit of all patients.
In November 2020, AACR Project GENIE marked the fifth anniversary of its public launch. The project has made extraordinary progress over the past five years, including several milestones in 2020:
- In December 2019, more than 44,000 cases from the project’s first four data releases were made available in the NCI Genetic Data Commons for combination with other cancer studies. The release was the result of an extensive collaboration between the NCI and the Project GENIE staff, which resulted in the development and publication of a tool to map data elements between multiple classification systems.
- In January 2020, one of the first clinical studies using GENIE data was published in the AACR journal Cancer Discovery. The data were initially presented at the AACR Annual Meeting 2019.
- At the virtual AACR Annual Meeting in April, a special session featured presentations of six proffered abstracts using the GENIE data, as well as an interim data analysis from the BPC NSCLC cohort. Chaired by Greg Riely, MD, PhD, the livestreamed session was viewed by more than 5,000 attendees.
- The seventh and eighth data releases in January and July 2020, respectively, increased the size of the registry to nearly 96,000 sequenced tumors from 100 major cancer types, including data from more than 14,000 patients with lung cancer, nearly 12,000 patients with breast cancer, and nearly 9,500 patients with colorectal cancer.
As the year ended, the Project GENIE team prepared for its ninth release in January 2021, which will increase the size of the registry to more than 100,000 samples.
AACR Hematologic Malignancies Task Force
Chaired by Jonathan D. Licht, MD, the AACR Hematologic Malignancies Task Force was formed to guide the AACR’s programs and initiatives dedicated to blood-based cancers. In 2020, the task force worked to expand the AACR's hematologic malignancy programs. Its members made recommendations on blood-based cancer sessions to the Annual Meeting program committee, and they laid the groundwork for future AACR conferences on acute myeloid leukemia/myelodysplastic syndrome and pediatric hematologic malignancies. The programs and initiatives launched by the task force have generated great interest among the hematological malignancies research community, as more than 3,700 new members with a focus on blood-based cancers have joined the AACR in the last two years.
AACR Pathology Task Force
Under the leadership of chair Massimo F. Loda, MD, the AACR Pathology Task Force is charged with helping the AACR understand the needs of current pathologists, increasing the visibility of pathology-focused research, integrating pathology researchers into AACR activities, and developing the next generation of pathologists. In 2020, the task force laid the groundwork for future scientific programs focused on pathology. Its members provided recommendations on pathology sessions to the program committees of the 2020 and 2021 AACR Annual Meetings—including the development of an educational session at the virtual meeting, “The Evolving Role of the Pathologist in Cancer Research.” The session highlighted emerging digital imaging technologies and computational tools, including artificial intelligence, which pathologists are utilizing to improve both clinical practice and cancer research. The task force built upon the topics of that session while supporting the development of a new AACR special conference, “Artificial Intelligence, Diagnosis, and Imaging,” which will take place in January 2021.
SITC-AACR Workshop: The Cancer Biology Underlying Immunotherapy-Induced Autoimmunity
Held in March 2020, this joint workshop between the AACR and the Society for Immunotherapy of Cancer (SITC) was chaired by SITC Past President Lisa H. Butterfield, PhD, AACR Past President Elizabeth M. Jaffee, MD, FAACR, and Arlene H. Sharpe, MD, PhD, FAACR. It convened expert scientists and clinicians to address cancer immunotherapy toxicities and advance our understanding of the science behind these toxicities. SITC and the AACR agreed to publish a joint white paper to codify the conclusions and recommendations of the workshop and to provide a blueprint for future collaborations.
Science Education, Career Development, and Continuing Medical Education
Continued progress against cancer necessitates a robust pipeline of scientists and clinicians to populate the cancer workforce. The AACR and its Science Education and Career Advancement Committee (Kathleen W. Scotto, PhD, chair) sustains this pipeline through its science education and professional development programs, supporting current and emerging investigators at all stages of their careers.
Fostering the Next Generation of Cancer Researchers
Despite the challenges of the pandemic, the AACR found ways to inspire high school and undergraduate students who are considering careers in cancer research. AACR staff supported high school science fairs in and around the AACR’s headquarters city of Philadelphia, awarding prizes to six students who presented meritorious projects. In addition, although the termination of the in-person Annual Meeting curtailed the undergraduate program, the committee still presented 21 undergraduate students with AACR Undergraduate Scholar Awards for participating in the virtual AACR Annual Meeting.
Continuing Medical Education (CME)
The AACR was reviewed by the Accreditation Council for Continuing Medical Education (ACCME®) and awarded Accreditation with Commendation for six years as a provider of continuing medical education (CME) for physicians (through March 2024). The AACR offered CME credit at 15 different meetings in 2020, including six special conferences, four joint conferences, one joint providership meeting, one joint providership regularly scheduled series, and the AACR Virtual Annual Meeting II. AACR journals provided another educational resource, offering credit to investigators for reviewing manuscripts. New this year was an on-demand activity collaboration called the FDA-AACR Project Livin’ Label. A total of 3,197 researchers and clinicians claimed CME credit from the AACR in 2020, and an additional 203 physicians obtained Medical Knowledge Maintenance of Certification (MOC) points through the American Board of Internal Medicine’s (ABIM) MOC program.
Meetings and Educational Workshops
By the time the COVID-19 outbreak forced the termination of in-person meetings in March 2020, the AACR had already hosted eight cutting-edge scientific conferences. While the disruption caused by the pandemic ultimately forced the cancellation of several AACR programs, the program chairs, the special conferences committee, and the AACR staff moved quickly to shift most of the slate of AACR conferences to a virtual format. Thanks to their efforts—and the efforts of the 15 scientific organizations with which the AACR collaborated to develop programs—the AACR convened a total of 29 meetings and workshops in 2020. These meetings advance the mission of the AACR by defining and advancing the frontiers of innovative cancer science.
A critical example of these innovation-focused programs was the AACR Conference, “Advancing Precision Medicine Drug Development: Incorporation of Real-World Data and Other Novel Strategies.” Held in January, this first AACR meeting of 2020 presented a comprehensive vision of the state of the art in precision medicine—including traditional topics such as trial design, diagnostics, and drug development; technology topics such as real-time patient monitoring, electronic health records, and machine learning; and regulatory and financial considerations. Under the leadership of cochairs David M. Hyman, MD, Elaine R. Mardis, PhD, FAACR, Lillian L. Siu, MD, and Eliezer M. Van Allen, MD, the meeting brought together scientists and clinicians with leaders from the insurance, finance, and technology sectors to explore how advances in biomedical research, technology, the interrogation of real-world data, and health care delivery could impact precision medicine and patient care.
2020 SCIENTIFIC PROGRAMS
- ADVANCING PRECISION MEDICINE DRUG DEVELOPMENT: INCORPORATION OF REAL-WORLD DATA AND OTHER NOVEL STRATEGIES
January 9–12 San Diego, California
Conference Cochairs: David M. Hyman, MD, Elaine R. Mardis, PhD, FAACR, Lillian L. Siu, MD, and Eliezer M. Van Allen, MD

- SIXTH AACR-IASLC INTERNATIONAL JOINT CONFERENCE: LUNG CANCER TRANSLATIONAL SCIENCE FROM THE BENCH TO THE CLINIC
January 11–14; San Diego, California
Conference Chairs: John V. Heymach, MD, PhD, and Katerina A. Politi, PhD
Conference Cochairs: Trever G. Bivona, MD, PhD, and Christine M. Lovly, MD, PhD
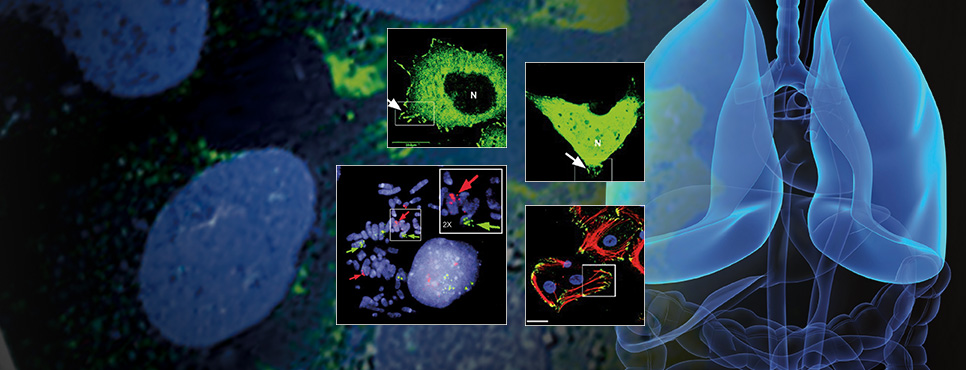
- ADVANCES IN LIQUID BIOPSIES
January 13–16, Miami, Florida
Conference Cochairs: Luis A. Diaz Jr., MD, Maximilian Diehn, MD, PhD, Irene M. Ghobrial, MD, and Nicholas C. Turner, MD, PhD

- FDA-AACR WORKSHOP TO EXAMINE UNDERREPRESENTATION OF AFRICAN AMERICANS IN MULTIPLE MYELOMA CLINICAL TRIALS
February 13; Washington D.C.
Workshop Cochairs: Kenneth C. Anderson, MD, FAACR, Nicole J. Gormley, MD, Paul G. Kluetz, MD, and Lola A. Fashoyin-Aje, MD
- THE MICROBIOME, VIRUSES, AND CANCER
February 21–24; Orlando, Florida
Conference Cochairs: Cynthia L. Sears, MD, Giorgio Trinchieri, MD, Jennifer A. Wargo, MD, and Laurence Zitvogel, MD, PhD

- FDA-AACR-IASLC WORKSHOP TO ADDRESS THE CRITICALITY OF TOBACCO USE ASSESSMENT IN ONCOLOGY THERAPEUTIC TRIALS
February 28; Silver Spring, Maryland
Cochairs: Roy S. Herbst, MD, PhD, Michael E. Menefee, MD, and Matthew Steliga, MD
- THE EVOLVING LANDSCAPE OF CANCER MODELING
March 2–5; San Diego, California
Cochairs: Cory Abate-Shen, PhD, Andrea Califano, PhD, Jos Jonkers, PhD, and Calvin J. Kuo, MD, PhD
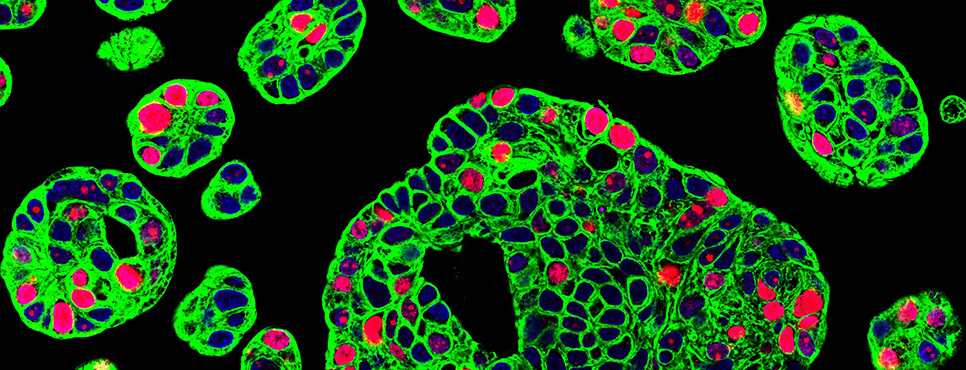
- EACR-AACR BASIC AND TRANSLATIONAL RESEARCH CONFERENCE: THE TUMOR MICROENVIRONMENT IN PARTNERSHIP WITH ASPIC (PORTUGUESE ASSOCIATION FOR CANCER RESEARCH)
March 2–4; Lisbon, Portugal
Scientific Committee Cochairs: Carlos M. Caldas, MD, PhD, Luís Costa, MD, Lisa M. Coussens, PhD, FAACR

- NIH-AACR CANCER, AUTOIMMUNITY, AND IMMUNOLOGY CONFERENCE
March 23–24; Virtual
Organizing Committee Cochairs: Julie R. Brahmer, MD, Elad Sharon, MD, MPH, Howard Young, PhD, Katarzyna (Kasia) Bourcier, PhD, Marie Mancini, PhD, Annette Rothermel, PhD, and Lisa Spain, PhD
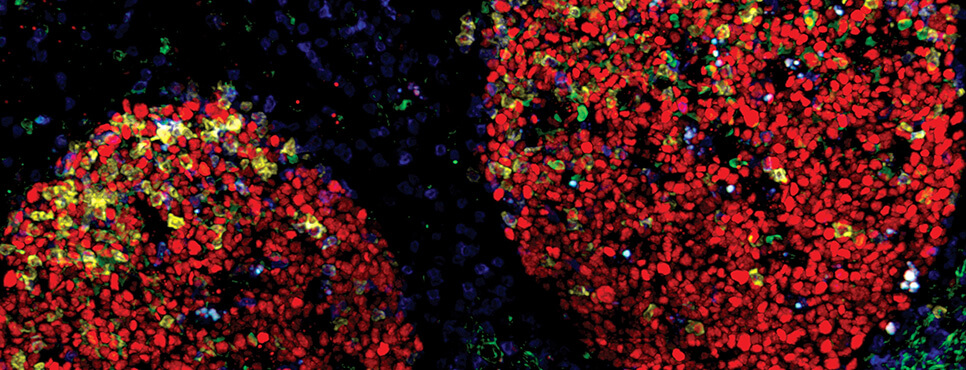
- AACR VIRTUAL ANNUAL MEETING I
April 27–28; Virtual
Program Committee Chair: Antoni Ribas, MD, PhD, FAACR
- AACR VIRTUAL ANNUAL MEETING II
June 22–24; Virtual
Program Committee Chair: Antoni Ribas, MD, PhD, FAACR
- COVID-19 AND CANCER
July 20–22; Virtual.
Organizing Committee Chair: David A. Tuveson, MD, PhD, FAACR
- ADVANCES IN MALIGNANT LYMPHOMA: MAXIMIZING THE BASIC-TRANSLATIONAL INTERFACE FOR CLINICAL APPLICATION
In cooperation with the International Conference on Malignant Lymphoma (ICML)
August 17–19; Virtual
Scientific Committee Chair: Ari M. Melnick, MD
- VIRTUAL OVARIAN CANCER RESEARCH SEMINAR SERIES
Seminar 1: September 3
Seminar 2: September 10
Seminar 3: September 17
Seminar 4: September 24
Virtual
Presented by the Rivkin Center and the American Association for Cancer Research
Planning Committee Cochairs: Douglas A. Levine, MD, Ursula A. Matulonis, MD, Barbara Norquist, MD, and Kunle Odunsi, MD, PhD

- TUMOR HETEROGENEITY: FROM SINGLE CELLS TO CLINICAL IMPACT
September 17–18; Virtual
Conference Cochairs: Nicholas E. Navin, PhD, Kornelia Polyak, MD, PhD, FAACR, Alex K. Shalek, PhD, and Charles Swanton, MD, PhD, FAACR
- PANCREATIC CANCER
September 29–30; Virtual
Conference Cochairs: Dafna Bar-Sagi, PhD, FAACR, Elizabeth M. Jaffee, MD, FAACR, Ben Z. Stanger, MD, PhD, and Brian M. Wolpin, MD, MPH
- THE SCIENCE OF CANCER HEALTH DISPARITIES IN RACIAL/ETHNIC MINORITIES AND THE MEDICALLY UNDERSERVED
In association with the AACR Minorities in Cancer Research Council
October 2–4; Virtual
Chair: John D. Carpten, PhD

- EPIGENETICS AND METABOLISM
October 15–16; Virtual
Cochairs: Chi Van Dang, MD, PhD, FAACR, Kimberly Stegmaier, MD, Craig B. Thompson, MD, FAACR, and Matthew G. Vander Heiden, MD, PhD
- TUMOR IMMUNOLOGY AND IMMUNOTHERAPY
October 19–20; Virtual
Cochairs: Timothy A. Chan, MD, PhD, Charles G. Drake, MD, PhD, Marcela V. Maus, MD, PhD, and Arlene H. Sharpe, MD, PhD, FAACR
- INNOVATION AND BIOMARKERS IN CANCER DRUG DEVELOPMENT: A JOINT MEETING PRESENTED BY THE EORTC, NCI, EMA, AND AACR
October 23; Virtual
Cochairs: Roberto Salgado, MD, PhD, Tracy G. Lively, PhD, Sinan B. Sarac, MD, MSc, PhD, and David B. Solit, MD

- EORTC-NCI-AACR MOLECULAR TARGETS AND CANCER THERAPEUTICS SYMPOSIUM
October 24–25; Virtual .
Scientific Committee Cochairs: Emiliano Calvo, MD, PhD, James L. Gulley, MD, PhD, and William R. Sellers, MD

- SBOC-AACR JOINT CONGRESS: A TRANSLATIONAL APPROACH TO CLINICAL ONCOLOGY
October 29–30; Virtual
Scientific Program Committee Cochairs: Carlos Gil M. Ferreira, MD, PhD, Manuel Hidalgo, MD, PhD, Paulo Marcelo G. Hoff, MD, PhD, and Sergio D. Simon, MD, PhD
- FRONTIERS IN CANCER SCIENCE
November 2-6; Virtual
Organizing Committee Chair: David M. Virshup, MD

- ENDOMETRIAL CANCER: NEW BIOLOGY DRIVING RESEARCH AND TREATMENT
November 2–6; Virtual
Cochairs: Victoria L. Bae-Jump, MD, PhD, Blake Gilks, MD, and Douglas A. Levine, MD
- SAN ANTONIO BREAST CANCER SYMPOSIUM
December 8–11; Virtual
Codirectors: Carlos L. Arteaga, MD, FAACR, Virginia G. Kaklamani, MD, and C. Kent Osborne, MD
2020 EDUCATIONAL WORKSHOPS
- ACORD—AUSTRALIA & ASIA PACIFIC ONCOLOGY RESEARCH DEVELOPMENT WORKSHOP
September 27–October 2, 2020; Virtual
Convenor: Martin Stockler, MBBS(Hons) MSc(Clin Epi), University of Sydney, Sydney, Australia
